Ich Q10 Pharmaceutical Quality System
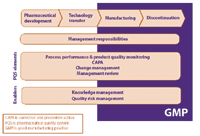
Ich q10 pharmaceutical quality system. And Strengthen the link between pharmaceutical development and manufacturing organisations. ICH Q10 is a model for a pharmaceutical quality system that can be implemented throughout the different stages of a product lifecycle. QMS required by GMP do not cover the full life cycle.
Submit Comments You can submit online or written comments on. The page is under construction. Continual Improvement of Process Perfomance and Product QualityPerfomance and Product Quality Chapter 4.
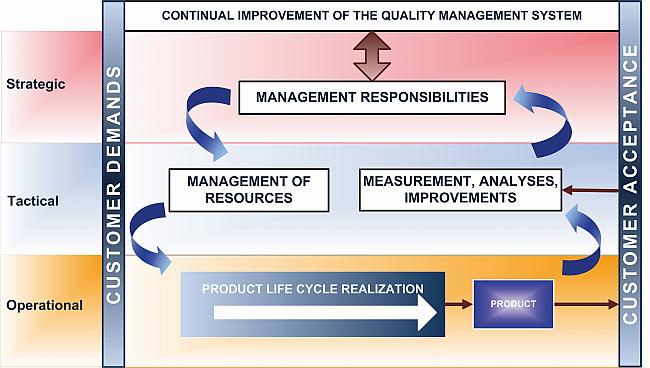
Management system to direct and control a pharmaceutical company with regard to quality. Innovation and continual improvement throughout the product lifecycle. ICH Q10 is a model for a pharmaceutical quality system that can be implemented throughout the different stages of a product lifecycle.
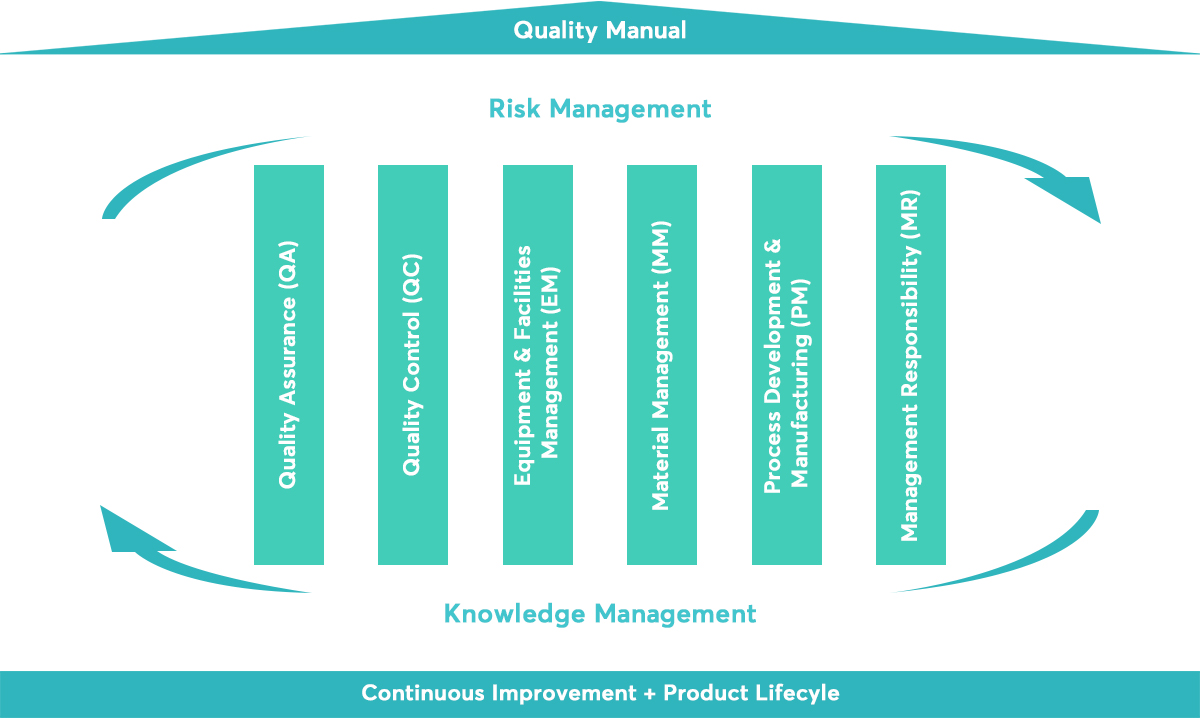
ICH Q10 Guideline deals with Pharmaceutical Quality System which guides about Quality Manual Management Commitment Quality planning Quality Policy Quality risk management. ICH Guideline Q10 on Pharmaceutical Quality System. In 2008 ICH Q10 Pharma Quality System PQS was designed to help improve public health around the world by enhancing the quality and availability of medicine.
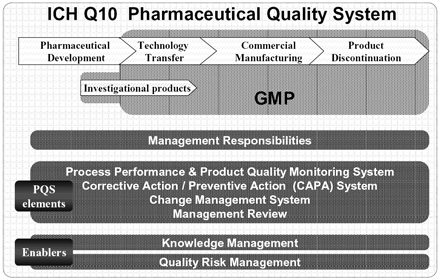
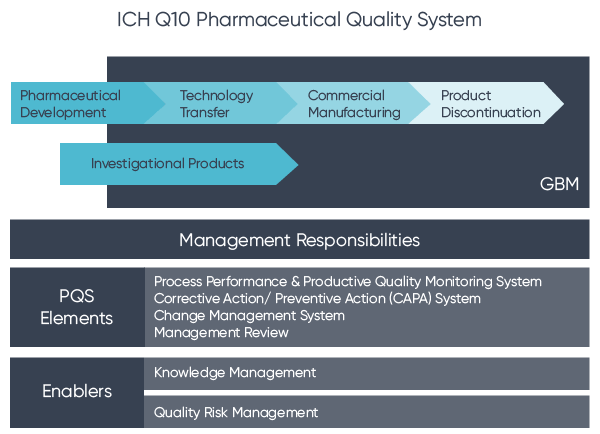
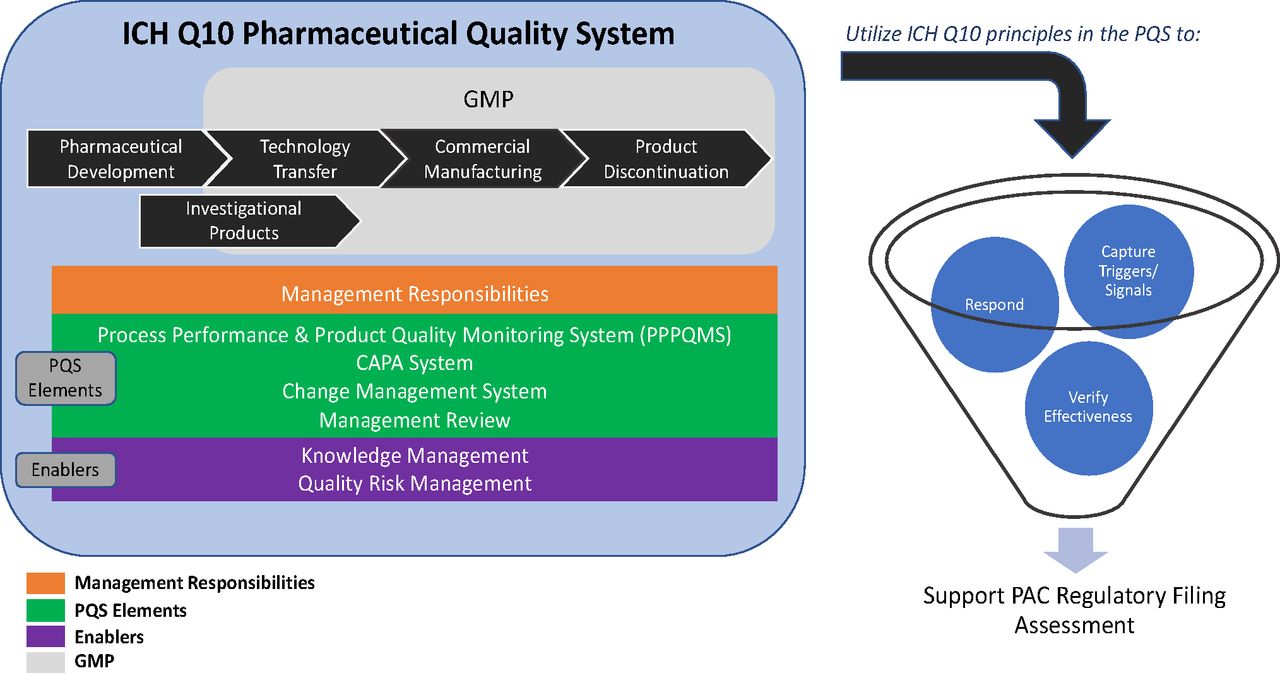
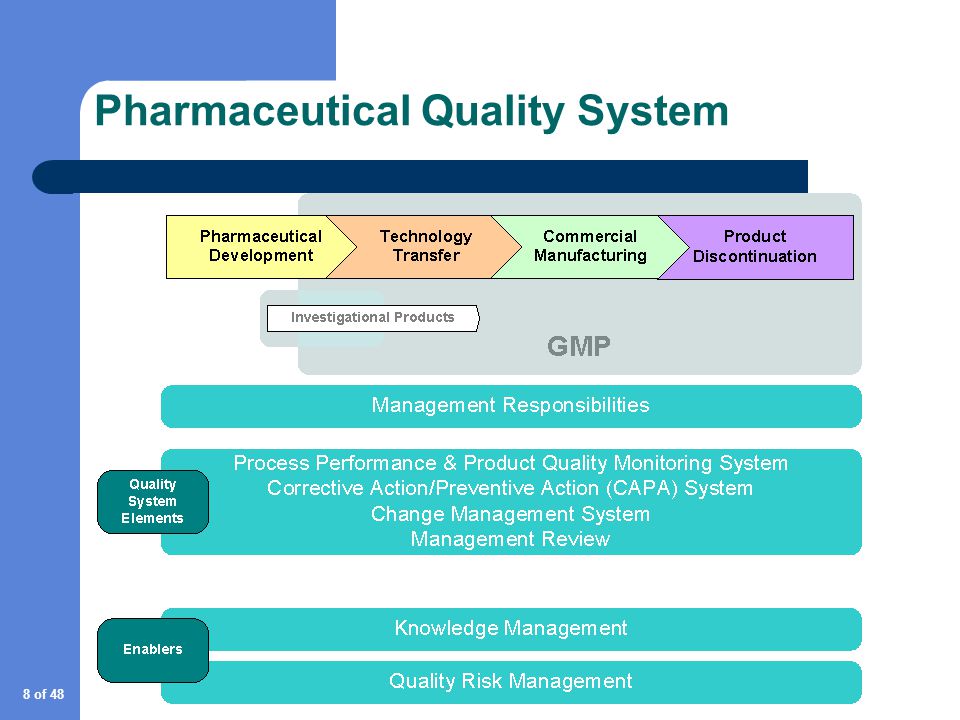
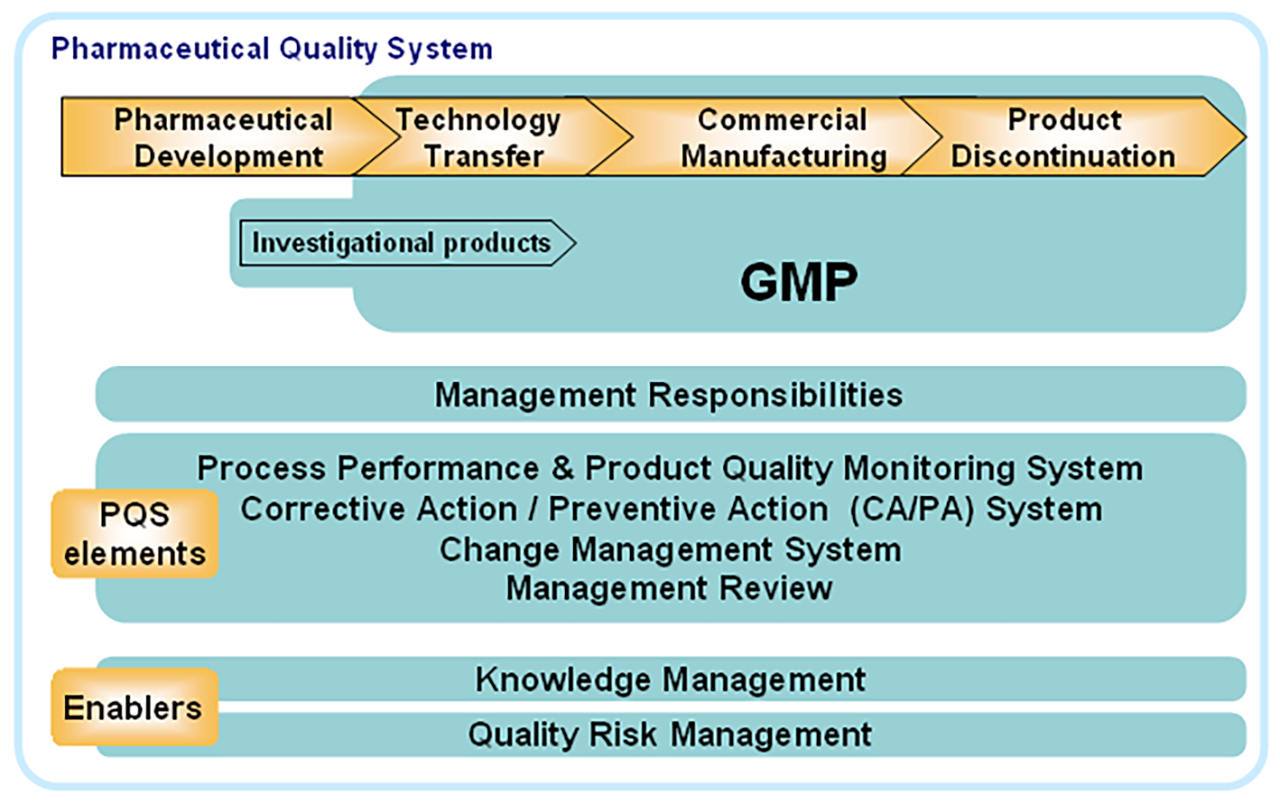
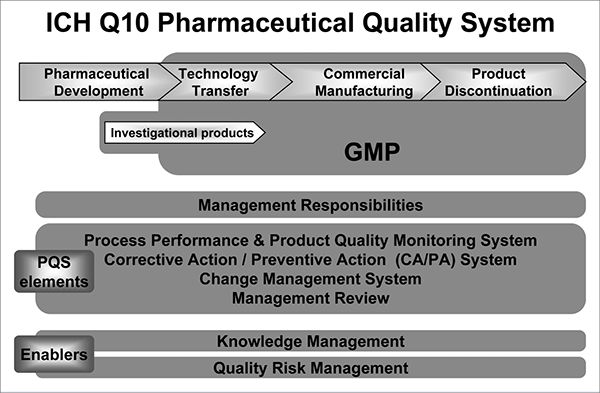
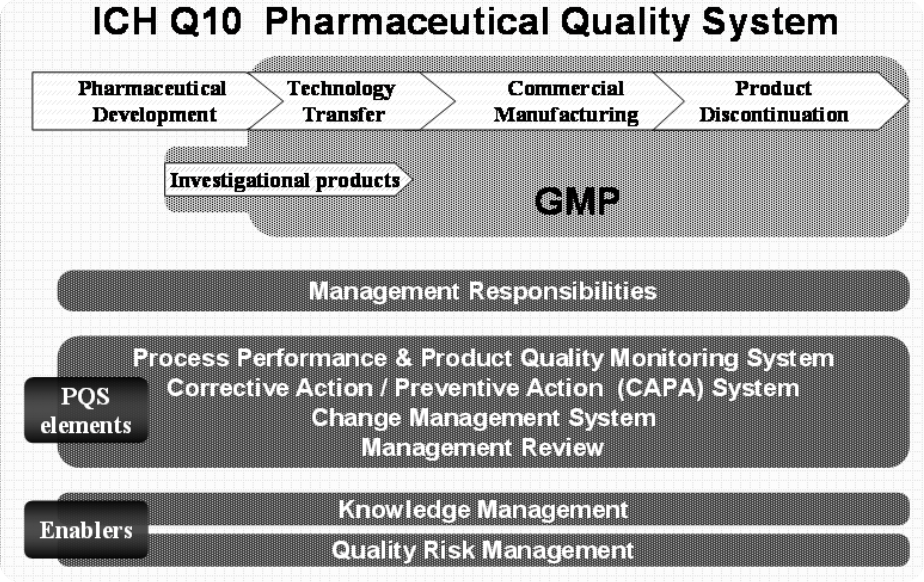
This document describes a model for an effective quality management system. Pharmaceutical quality system refers to the ICH Q10 model. ICH Q10 Pharmaceutical Quality System PQS.
ICH Q10 was designed to facilitate the efficient delivery of high-quality products to people who need them most. Complaints and Recalls. The goal is to harmonize post-approval changes to facilitate scientific innovation and help to mitigate drug shortages.
The ICH comprises professionals from both the manufacturing sector and regulatory bodies. Throughout this guidance the term pharmaceutical quality system refers to the ICH Q10 model.
Management ResponsibilitiesManagement Responsibilities Chapter 3.
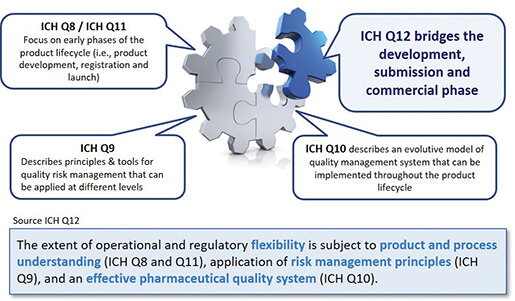
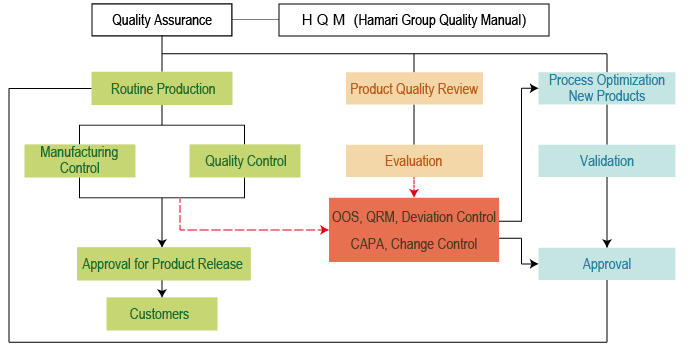
ICH Q10 - Corrective and Preventive Action. ICH Q10 based upon ISO 90002005 12 13. ICH Q10 describes one comprehensive model for an effective pharmaceutical quality system that is based on International Standards Organisation ISO quality concepts includes applicable Good Manufacturing Practice GMP regulations and complements ICH Q8 Pharmaceutical. Continual Improvement of Process Perfomance and Product QualityPerfomance and Product Quality Chapter 4. This document describes a model for an effective quality management system. Links development and manufacturing through product lifecycle Facilitates application of ICH Q8 Q9 Facilitates continual improvement in pharmaceutical manufacturing. Throughout this guidance the term pharmaceutical quality system refers to the ICH Q10 model. Much of the content of ICH Q10 applicable to manufacturing sites is currently specified by regional GMP requirements. Complaints and Recalls.
The ICH Guideline Q12 on technical and regulatory considerations for pharmaceutical product lifecycle management was published in January 2020. Diagram of the ICH Q10 Pharmaceutical Quality System Model. The FDA adopted ICH Q10 for A Process Approach to Pharmaceutical Quality Systems walks you step by step Please note the following procedure guidelines. ICH Q10 Pharmaceutical Quality System Complements existing GMPs GMP not a full QS. The ICH Q10 concepts will be presented in a logical sequence that will show their applicability to the pharmaceutical product lifecycle to strengthen the link between. Much of the content of ICH Q10 applicable to manufacturing sites is currently specified by regional GMP requirements. And Strengthen the link between pharmaceutical development and manufacturing organisations.



























Post a Comment for "Ich Q10 Pharmaceutical Quality System"